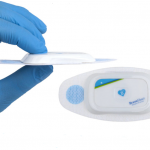
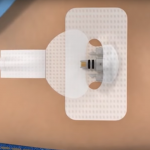
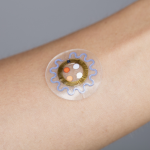
BraveHeart Wireless Receives FDA Clearance for its Life Sensor Cardiac Monitoring system
BraveHeart Wireless Inc., a New Hampshire-based leading innovator in clinical-quality biometric wearables, received FDA clearance for its BraveHeart Life Sensor Cardiac Monitoring system.
Related Stretchable...
Graphene-Based Sensors Detect Diabetic Foot Ulcers Before They Cause Injury
More than 100 million U.S. adults are now living with diabetes or prediabetes, according to a new report by the Centers for Disease Control...
Livongo Integrates Chronic Disease Management Service with Leading Smartwatches
Livingo, a chronic disease management platform, is now available in smartwatches, including leading models from Apple, Fitbit, and Samsung. Livongo Members can now connect...
Diagnostic Wearable Medical Devices Market Anticipated to Grow Substantially Through 2025
The global diagnostic wearable medical devices market is anticipated to grow substantially during the forecast period of 2019-2025.
Related Wearable Skin Patches Market to Grow...
Wearable Skin Patches Market to Grow to Over $20 Billion Per Year by 2029,...
Smart skin patches are widely used in wearable devices. These patches use laminar batteries, often partially printed, plus printed electrode patterns to deliver drugs,...
AMA Passes New Policy Recommendations Around Use of Augmented Intelligence
The American Medical Association (AMA) has passed a policy addressing Augmented Intelligence – and not Artificial Intelligence – that provides recommendations for stakeholders' concerns....