Insulet Corporation has announced that its Omnipod DASH Insulin Management System has received 510 (K) clearance from the U.S. Food and Drug Administration (FDA). The clearance will allow Insulet to market Omnipod DASH in the United States, though Insulet has announced that it will start roll-out through a limited market launch for the first six months, starting in July.
Read more Valeritas’ V-Go Wearable Insulin Delivery Device Shows Promise for Type 2 Diabetes Patients
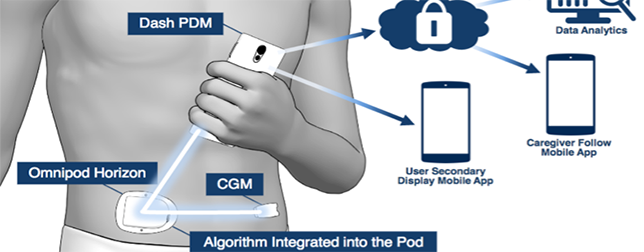
Omnipod DASH was built to be simple, discreet and easy-to-use. The system contains a tubeless stick-on waterproof insulin pump (Pod) that stays hidden under the skin, and controlled wirelessly without needing to lift the bottom of the shirt.
The accompanying smartphone-like device interfaces with the pump and monitors readings and amount of insulin delivered over time. The Bluetooth wireless technology connects between the color touch-screen Personal Diabetes Manager (PDM). If used along with the CONTOUR NEXT ONE blood glucose reader, the system adjusts the delivery of insulin accordingly by seamlessly keeping track of blood sugar levels and transferring them to PDM’s bolus calculator.
The company is offering DASH PDM at no cost with the purchase of Pods.
Doctor or family members of the patient can access the data coming from the glucometer and insulin pump, helping them to ensure proper usage and compliance, and also prevent hazardous glucose levels.
“Omnipod DASH was inspired by Podders and embodies what users on multiple daily injections have been asking for in a diabetes management system,” said Patrick Sullivan, Chairman and CEO of Insulet. “Our number one priority is to continue to minimize the daily strain on those impacted by diabetes and we are confident this system, and eliminating the system’s upfront cost, do just that.”
Read more JDRF Partners with Korean Company to Develop Wearable Insulin Pump
Tessa Mellinger, a college student with Type 1 diabetes was part of the DASH user testing and development. She said, “I’m thrilled with the new Omnipod DASH System. The PDM display is easy to read, the steps to managing basal rates are user friendly, and the food library is really helpful. The fact that it looks like a smartphone makes it cool to carry and may help eliminate those awkward questions from strangers.”
The system represents the latest technology update pushing Omnipod DASH closer to the Horizon Hybrid Closed Loop System currently being developed in clinical trials.
During the first six months of limited launch, DASH PDMs will be made available through a select group of endocrinologists participating in the “Omnipod DASH AHEAD program”.
Full market release of the system is expected to begin in early 2019 in the United States.
Find more innovative healthcare solutions at our WT | Wearable Technologies Show 2018 MEDICA on November 12-15.