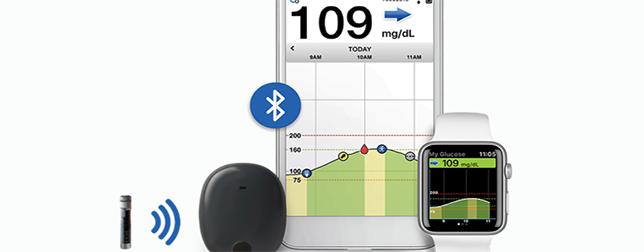
A device that can open a new chapter in diabetes technology has been approved by the FDA. Last week, the FDA cleared way for Eversense, a Continuous Glucose Monitoring (CGM) system that can be implanted underneath the skin in the arm. Unlike traditional CGMs that need cannula insertion, and are worn outside the body for up to 10 days before requiring replacement, the tiny device the size of a small pill can be implanted onto the skin for 90 days.
Read more Insulet Wins FDA Approval for Omnipod DASH Insulin Management System
“The FDA is committed to advancing novel products that leverage digital technology to improve patient care,” FDA Commissioner Dr. Scott Gottlieb said in a statement. “These technologies allow patients to gain better control over their health. This approval of a more seamless digital system that gives patients the ability to effectively manage a chronic disease like diabetes is a vivid illustration of the potential for these mobile platforms.”
Over 30 million people (9.4% of the population) have diabetes in the US, according to a 2017 CDC report. Over 7 million diabetics remain undiagnosed, and for adults, the risks for getting diabetes increases significantly with age. Among people aged 65 or over, more than 24% have diabetes, and millions more are impacted by the struggle to control their glucose levels.
A landmark 2015 analysis of 46 different type 2 diabetes studies found hypoglycemia to be especially prevalent among those taking insulin, affecting 50 percent of those with diabetes averaging 23 incidents per year.
Senseonics President and CEO Tim Goodnow said the approval took a little while because the implantable CGM is novel and the FDA chose to convene a panel meeting of medical experts.
This new clearance applies to an older version of Senseonics device, which covers the 90-day monitoring model, whereas the version in the European markets lasts for 180 days.
Read more LifePlus Announces First Noninvasive Continuous Glucose Monitoring Wearable
The Eversense CGM System comprises of a fluorescence-based sensor, a Bluetooth transmitter for data communication and a mobile app that displays glucose levels, trends and alerts. The sensor lasts up to 3 months and needs to be inserted subcutaneously in the upper arm by a physician in a brief in-office procedure. Traditional CGMs require patients to self-administer insertions of the sensors weekly or biweekly. Additionally, CGM’s transmitter can be removed and recharged without discarding the sensor.
Unlike in Europe, where Senseonics has commercialized its devices through a partnership with Roche, in the United States it will sell directly to endocrinologists.
Goodnow said his company expects to have the first device in patients’ hands by the end of July.