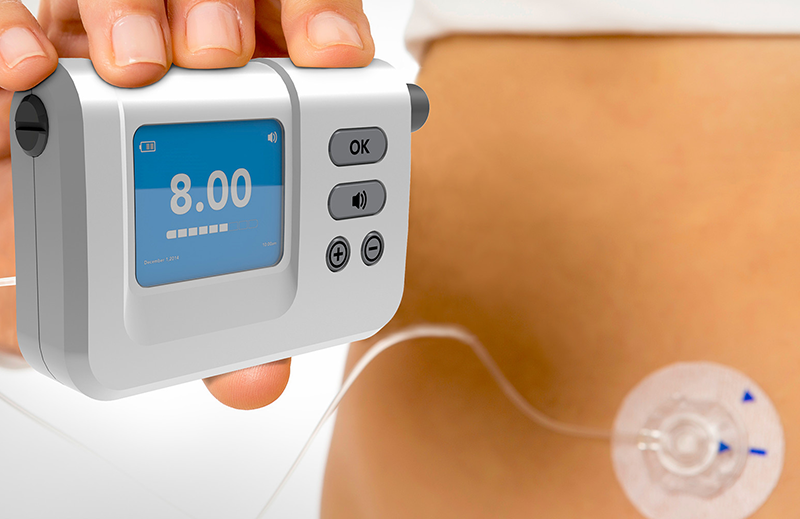
Wearable devices are changing the way drugs are delivered. The size of the market opportunity for Large Volume Wearable Injectors (LVIs) alone has been estimated at $8.1 billion by 2025, with over 50% of this driven by devices to deliver drugs for cancer and associated conditions.
With the commercialization of the first wearable self-injection system – Amgen’s Repatha (evolocumab) – the landscape for injectable medication has changed. West’s first-generation SmartDose system, in the form of the Pushtronex, was the first commercial wearable self-injection system on the market. Manufacturing Chemist discusses ways to create an effective drug delivery system.
Making a good first impression
The drug delivery system should be focused on ensuring ease of use for the patient through minimal steps and a convenient format. With more and more biologic drugs entering the market, delivery systems need to be able to accommodate a range of dose volumes and injection times or rates, while still satisfying the patients’ need for a small and simple system.
Read more How Smart Pills Could Revolutionize Healthcare
Insight along the development process
Different analytical studies can help to determine the proper primary containment selection, including the need for barrier film-coated elastomers or a cyclic olefin polymer container instead of glass.
The final selection of materials may help to map out delivery options. For example, Cyclic olefin polymers, like the Daikyo Crystal Zenith cartridge, can offer design flexibility as they can be molded into various shapes and sizes during the SmartDose drug delivery platform; they’re also highly break resistant when compared with glass.
During the clinical phases, as the molecule becomes more structured, the delivery system calls for speed, flexibility and expertise. For example, Phase I may require multiple injections from a vial containment system; but, by Phase II, dose volume and frequency, as well as patient considerations, may drive the need for a delivery system such as prefilled syringe, autoinjector or wearable injector. The ability to fill the container at different stages must also be considered.
As the drug enters commercialization phase, the manufacturer must consider patient adherence, onboarding and compliance with respect to drug delivery. Additionally, it is critical to consider what information is required to support product registration, including stability studies, human factor testing, device studies, etc. At this stage, it is also critical to consider the overall supply chain and provide a robust solution, including containers, drug, filling, device manufacture and assembly, secondary assembly of the device and drug container, patient services (training and adherence solutions) and a variety of other factors.
Read more Valeritas’ V-Go Wearable Insulin Delivery Device Shows Promise for Type 2 Diabetes Patients
Optimizing with patients in mind
The typical drug development cycle can be as long as several years. Improving drug containment and delivery systems — from primary containment to commercialization — can help to ensure that when pharmaceutical manufacturers are ready to move to market, the delivery system will make a great first impression on the patient. By understanding the requirements of the drug product as well as the patient, continued innovation in wearable injectors will help move products to market with minimal risk and maximum benefit.