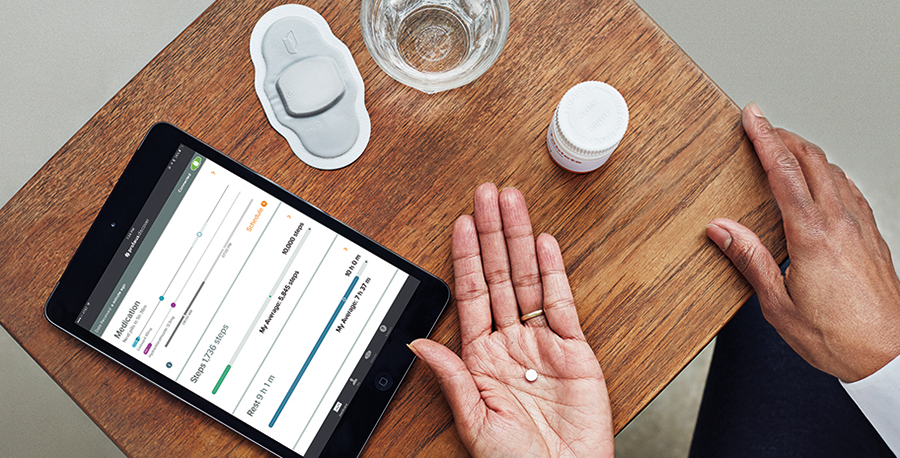
Otsuka Pharmaceutical Co., Ltd. and Proteus Digital Health has agreed to expand global collaboration for the further development and commercialization of a portfolio of medicines including the FDA-approved ABILIFY MYCITE® (aripiprazole tablets with sensor).
As part of the expanded collaboration, Otsuka made $88 million in related equity and other payments to Proteus. The expanded agreement covers the development and commercialization of digital medicines over the next five years, said a press release.
“We are pleased to continue to focus on opportunities to further integrate digital medicines into healthcare eco-systems to provide value-added outcomes for patients suffering from unmet medical needs in the mental health field,” stated Kabir Nath, President and CEO, Otsuka North America Pharmaceutical Business Division, Otsuka America, Inc. “Our expanding collaboration with Proteus is a cornerstone of this strategy, and further enables us to serve the mental health community by developing additional innovative technology solutions.”
A team of researchers from both Otsuka and Proteus will work together for the commercial development and market coordination of the ABILIFY MYCITE System, software integration and standardization, manufacturing and supply chain integration, and coordination. This includes the joint development of an expanded portfolio of digital medicines consisting of other therapies such as atypical antipsychotics used in the treatment of serious mental illness integrated with Proteus sensors.
The companies will together on the development of next generation product features and sensor capabilities to expand the potential of digital medicine offerings.
Andrew Thompson, President and CEO of Proteus Digital Health said this dedication to a wide inter-operable platform is the main reason his company expanding its collaboration with Otsuka in mental health. “This expanded collaboration is a great opportunity to bring novel digital medicine solutions to mental health patients,” he said.
Read more How Smart Pills Could Revolutionize Healthcare
About ABILIFY MYCITE
ABILIFY MYCITE® is FDA-approved aripiprazole tablets with sensor to track drug ingestion, for the treatment of schizophrenia, bipolar disorder, acute manic and mixed episodes and maintenance therapy, and the adjunctive treatment of major depressive disorder. ABILIFY MYCITE has not been shown to improve patient compliance or for use in modifying aripiprazole dosage. It should not be used in “real-time” or during an emergency, because detection may be delayed or not occur. The ABILIFY MYCITE System provides an objective summary of drug ingestion over time, to help enhance collaboration with healthcare providers who treat patients with certain serious mental illnesses. Only functions related to tracking drug ingestion have been evaluated or approved by FDA.