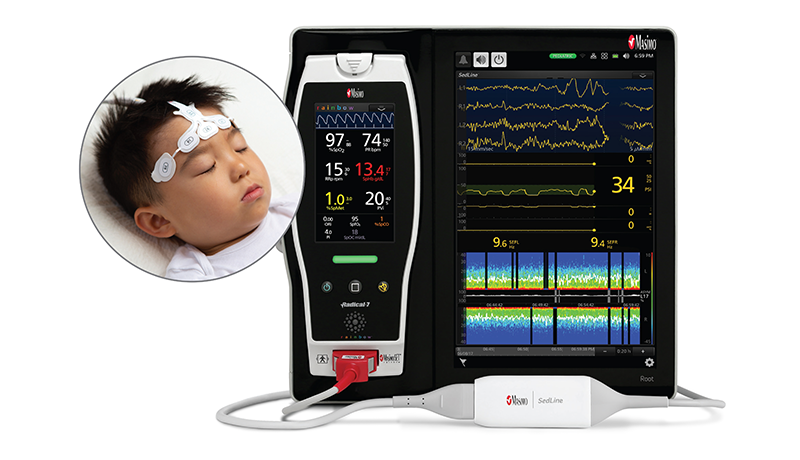
Masimo, an Irvine, California-based manufacturer of noninvasive patient monitoring technologies, received CE marking for its next generation SedLine® brain function monitoring for pediatric patients. Patients aged one year and above, living in CE mark countries, can now enjoy the benefits of Next Generation SedLine.
Read more Masimo’s Rainbow Acoustic Monitoring RAS-45 Breathing Sensor for Babies Gets FDA Clearance
SedLine helps clinicians monitor the state of the brain under anesthesia with bilateral data acquisition and processing of four leads of electroencephalogram (EEG) signals.
For patients one year old and above, SedLine uses a pediatric-specific signal processing engine to improve performance of Masimo’s processed EEG parameter, the Patient State Index (PSi). As monitoring anesthesia on pediatric patients is different than monitoring adults, it is important to maintain an appropriate level of anesthesia to prevent anesthesia-related events and speed-up recovery. The Psi in SEdLine is especially focused at helping clinicians interpret the EEGs of the pediatric population, reports Business Wire.

Other benefits of the Next Generation SedLine over the original SedLine include:
- A PSi with less susceptibility to electromyography (EMG) interference
- A Multitaper Density Spectral Array (DSA), which may enhance visibility of EEG features
“Next Generation SedLine is doing for brain function monitoring what Masimo SET® did for pulse oximetry. We believe Next Generation SedLine is the best and most advanced way to monitor depth of sedation, crucial to helping ensure patients with even the most challenging brains are appropriately anesthetized. We are gratified that its benefits are now available to those younger patients whose brains are particularly delicate and to whose wellbeing Masimo has always been so committed,” said Joe Kiani, Founder and CEO of Masimo.
Read more FDA Approves Seizure-Detecting Smartband Embrace 2 for Use by Children
Next Generation SedLine has received FDA clearance for adults but is not yet available for pediatric patients in the United States.