The US Food and Drug Administration (FDA) has issued a recall for Senseonics’ Eversense Continuous Glucose Monitoring (CGM) system.
Read more Senseonics & Glooko Partner Up for Integrated Diabetes Management
“Eversense Sensors have prematurely stopped functioning due to inadequate hydration of the sensor s glucose-sensing surface,” the FDA stated as the manufacturer reason for recall.
In September, Eversense posted a notice alerting providers and distributors in the US that the sensors of certain devices may stop working prematurely, reports MobiHealthNews.
“Our investigation found the presence of residual sodium chloride solution used during the manufacturing process from one specific manufacturer. In such cases, the Eversense CGM System stopped displaying glucose information to the user and ended sensor life earlier than expected (within first 3 weeks following insertion),” the company said.
The recall will affect 844 sensors that Eversense shipped between March 19 and August 19. “It does not affect systems marketed outside of the US, or the company’s Eversense Smart Transmitter, Eversense Insertion Kit or Eversense App,” according to MobiHealthNews report.

Senseonics said it knew that as of September 4, only 1.4% of the sensors (24 sensors) that were inserted into patients stopped functioning prematurely.
Read more Senseonics’ New Program Geared Towards Patients with High-Deductible Insurance Plans
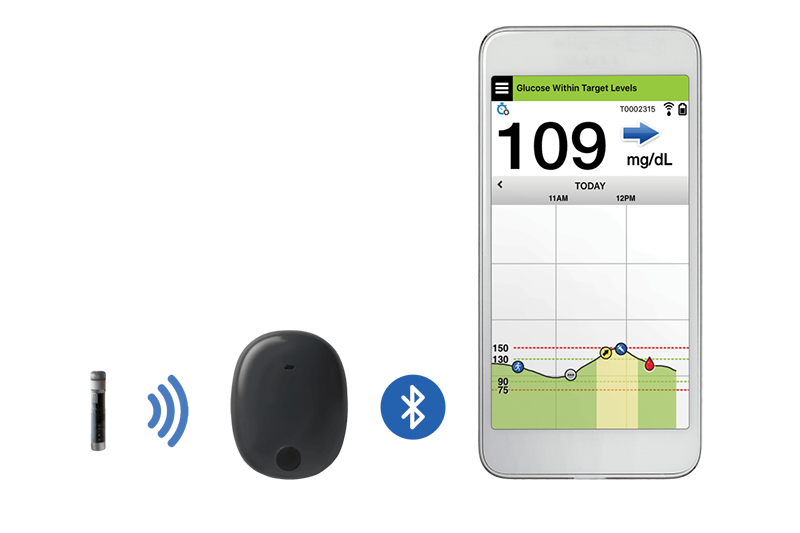
The Eversense CGM System comprises of a fluorescence-based sensor, a Bluetooth transmitter for data communication and a mobile app that displays glucose levels, trends and alerts. The sensor lasts up to 3 months and needs to be inserted subcutaneously in the upper arm by a physician in a brief in-office procedure. Traditional CGMs require patients to self-administer insertions of the sensors weekly or biweekly. Additionally, CGM’s transmitter can be removed and recharged without discarding the sensor.
The FDA and Senseonics asked providers not to use the devices on patients and return them to the company. The company also said it didn’t receive any report on injuries related to the faulty sensors.