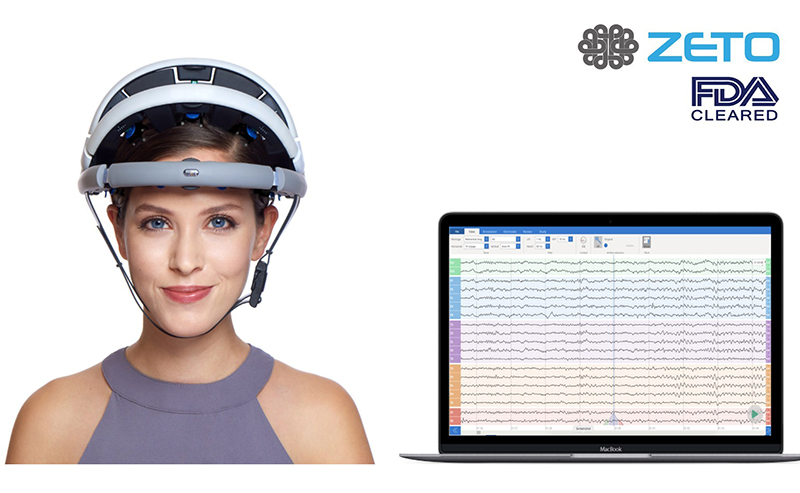
Zeto, a Santa Clara, CA-based medical technology company that is focused on transforming the way electroencephalography (EEG) is performed at hospitals and clinics, announced that it has raised $7.3 million in Series A funding. The funding round was led by Seraph Group and joined by Aphelion Capital, SV Tech Ventures and Shangbay Capital. Zeto said it will use the money to accelerate commercialization of its lead product, the zEEG headset and software platform, and develop new products to expand its portfolio.
Read more URGOnight Trains Your Brain During the Day So You Can Get Better Sleep at Night
zEEG is the first true dry electrode EEG headset to be approved by the U.S. Food and Drug Administration (FDA) for clinical use. The commercially available wireless EEG headset is backed by a cloud platform that offers instant upload, tools for analysis and remote interpretation by neurologist. It has 20 dry electrodes that can be positioned as per the 10-20 EEG system, says a press release.

“Since our commercial launch a few months ago, we added several customers who love the product and are utilizing it at high rates. We are glad to validate the strong product-market fit for zEEG. Inspired by the demand, we are excited to expand our commercial team and production capacity to serve our growing customer base,” said Aswin Gunasekar, MS, MBA, Chief Executive Officer and Founder of Zeto. “Our goal is to provide the most seamless and intelligent EEG experience for any clinical need. Current products are far behind what users need and Zeto is building a suite of convenient and intuitive products to eliminate the most time consuming and labor-intensive activities involved in traditional EEG. As a subsequent step, we plan to utilize artificial intelligence to not only supplement the reading physician with analytics and insight but also expand the diagnostic utility of EEG worldwide in areas of medicine where it has been underutilized.”
Read more Wearable EEGSmart UMindSleep Sleep Tracker Breaks Cover
Zeto’s customer, Wave Neuroscience, Inc., said, “Our extensive intellectual property portfolio and business operations are built on award-winning science and medical innovation. For us to achieve the high level of results we are committed to, we rely on partners that never rest in their quest to achieve the highest standards. Accessing an FDA compliant EEG device that performs at an uncompromising standard, allows for increased throughput and provides a user experience that rivals the latest technology assets, was very attractive to us. Zeto transformed the way we think about EEG capture by advancing technology further in the past two years than the industry had in the prior three or four decades. Zeto provides us the confidence, convenience and data quality that we rely on, day in and day out.”