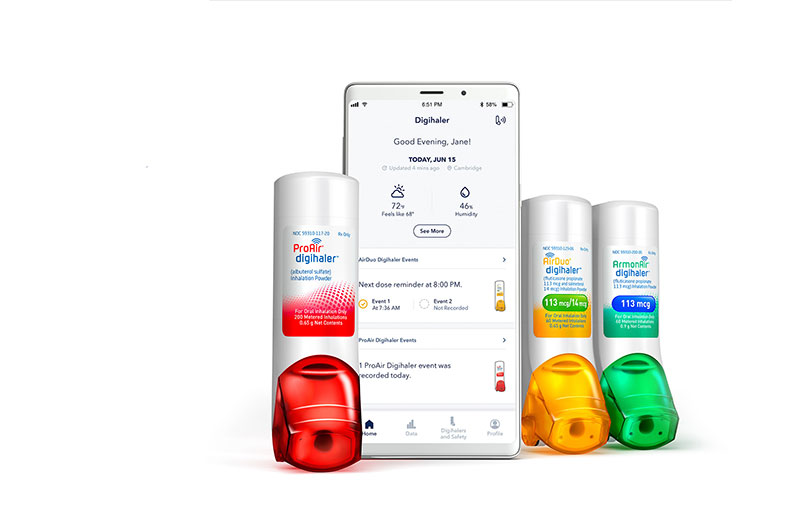
Tel Aviv, Israel-based pharmaceutical company Teva has launched 2 new digital maintenance inhalers, fluticasone propionate and salmeterol inhalation powder (AirDuo Digihaler) and fluticasone propionate inhalation powder (ArmonAir Digihaler), for patients with asthma.
AirDuo Digihaler is a prescription medicine used to control symptoms of asthma and to prevent symptoms such as wheezing in people 12 years of age and older. ArmonAir Digihaler is a prescription medicine for the long-term treatment of asthma in patients 12 years and older. AirDuo Digihaler and ArmonAir Digihaler are not used to relieve sudden breathing problems from asthma and will not replace the need for a rescue inhaler, reports BusinessWire.
“Being able to now offer AirDuo Digihaler and ArmonAir Digihaler to patients is an exciting step forward for Teva, and one that we are extremely proud of,” said Brendan O’Grady, Executive Vice President, North America Commercial at Teva Pharmaceuticals. “With the launch of these two maintenance products, we’re now able to offer the full Digihaler portfolio to patients, potentially allowing them to gain an even deeper understanding of their overall asthma treatment regimen due in part to the data collection capabilities of the Digihaler portfolio of products.”
AirDuo Digihaler was approved by the U.S. Food and Drug Administration (FDA) in July 2019 in a low, medium and high dose: 55/14 mcg, 113/14 mcg and 232/14 mcg administered as one inhalation twice daily. ArmonAir Digihaler was approved by the FDA in February 2020 in a low, medium and high dose: 55 mcg, 113 mcg and 232 mcg administered as one inhalation twice daily.

Both inhalers automatically detect, record and store objective inhaler use data, including peak inspiratory flow. Both products have the ability to remind the patient how often the devices have been used, measure inspiratory flow rates and determine if inhalation technique may need improvement.
“Until now, I have had to rely on my patients’ memory to share the details of their inhaler use habits with me – which, despite their best efforts, can be difficult and confusing,” said Dr. J. Allen Meadows, MD, FACAAI, Clinical Faculty, Alabama College of Osteopathic Medicine. “In addition, getting access into their inspiratory flow rates may also help me identify patients who might need coaching in inhaler technique improvement.”
Read more Apple Buys Asthma-Monitoring Startup Tueo Health
The Wholesale Acquisition Cost (WAC or “list price”) for AirDuo Digihaler in the doses 55/14 mcg and 113/14 mcg is $399 and the WAC price for the 232/14 mcg dose is $449. The WAC price of ArmonAir Digihaler in the doses 55 mcg and 113 mcg is $239 and the WAC price for the 232 mcg dose is $299. Actual costs to individual patients and providers for both products are anticipated to be lower than WAC.