Wearable sensing technology is an essential link in personalized medicine, where researchers must track multiple analytes inside the body simultaneously, to obtain a complete picture of human health. In a new report on Science Advances, Yingli Wang and a team of scientists in biosystems, engineering and information science at the University of Cambridge and Zhejiang University in the U.K. and China, presented a wearable plasmonic-electronic sensor with “universal” molecular recognition capability. The team introduced flexible plasmonic metasurfaces with surface-enhanced Raman scattering (SERS) activity as the fundamental sensing component. The system contained a flexible sweat extraction process to noninvasively extract and fingerprint analytes inside the body based on their unique Raman scattering spectra. As proof of concept, they successfully monitored varying trace-drug amounts inside the body to obtain an individual drug metabolic profile. The sensor bridged the gap in wearable sensing technology to provide a universal, sensitive molecular tracking process to assess human health.
Read more MIT Researchers Create Smart Fiber with Memory, Temperature Sensors, And Trained Neural Network
Wearable sensor technology
Wearable sensors must overcome a fundamental mismatch between a rigid and soft elastic surface to laminate into biointerfaces such as the skin, eye, nerve and tooth to seamlessly assess human health. The devices allow researchers to continuously assess vital signs including the heart rate and body temperature, perspiration and physical activities. Despite the success of physical wearable sensors, non-invasive molecule tracking techniques that provide insight into human body dynamics at the molecular level remain to be realized. These capabilities are vital for personalized precision medicine, reports phys.org.
The mechanism of action and the development of the sensor
The team fingerprinted the unique SERS spectrum using the wearable sensor. As a proof of concept, they detected the variation of drug concentrations in the human body to obtain an individual’s drug metabolic profile. The integrated wearable sensor bridged the existing gap in personalized diagnosis for real-time tracking of important biochemical compounds. The scientists used the sensing platform to monitor physiological cues or drug concentrations in the human body to obtain an individual’s drug metabolic profile. Then using the integrated wearable sensor, they monitored physiological cues or drug concentrations in a closed-loop feedback drug delivery system.
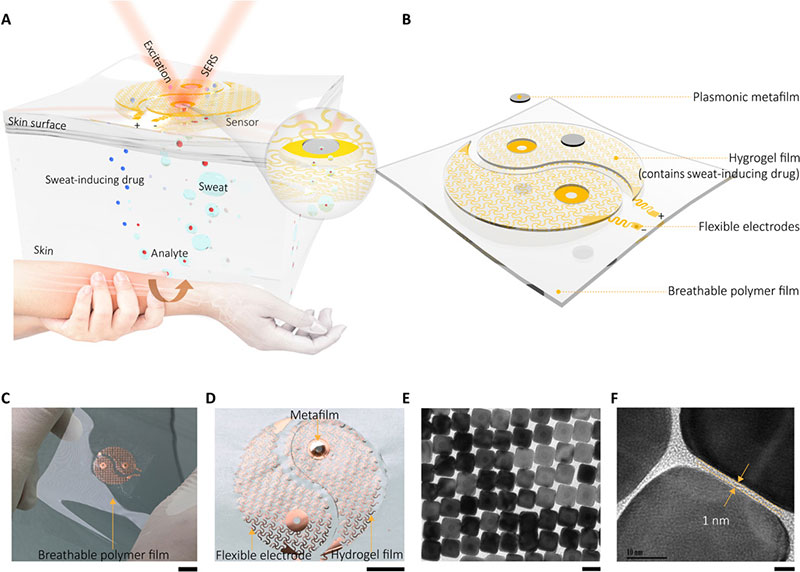
The plasmonic metamaterial-integrated wearable sensing device contained two major components including a thin layer of hydrogel loaded with molecules to stimulate sweat gland secretions. The team attached these constructs to two spiral fractal mesh electrodes to serve as the sweat extraction component. The researchers used the iontophoresis process (transdermal drug delivery) for this extraction; widely used as a non-invasive sweat sampling method in devices for diagnostic and therapeutic purposes.

They formed a plasmonic meta-film using an ordered silver nanocube superlattice to serve as the sensing component mounted in the experimental setup. The strong electromagnetic fields localized in the nanocube gave rise to the SERS (surface-enhanced Raman scattering) effect to detect molecules approaching the metafilm surface. They placed the two components on a thin ultralow-modulus polymer film to form a thin, breathable and physically tough support for nonirritating skin adhesion. Using the electrodes, the team applied a mild electric current to deliver acetylcholine chloride in the hydrogel layer to secretory sweat glands for rapid, localized sweat generation.
Biological sensing application
Next, Wang and her colleagues recruited healthy volunteers for in vivo (physiological) measurements to demonstrate the sweat extraction capability of the device. The scientists used nicotine as the model drug and monitored the actual concentration of the drug in skin relative to drug delivery, uptake and metabolic rate per individual. During the experiments they used a wearable SERS sensor coupled to a compact power supply and wireless control unit on the forearm of the volunteers. The device showed the SERS spectrum of nicotine in the sweat to match the spectrum of the nicotine standard. The results indicated how the sensor trained the metabolic behavior of nicotine to allow the wearable sensor’s capability to monitor the dynamic pharmacokinetics of drugs and their metabolic profile. The sensor, however, only effectively detected targets stored in the shallow sub-epidermis; therefore, the researchers will need to understand how this value correlates with drug concentrations in blood or interstitial fluid during further studies.
Read more Wearable Sensor Collects Data from Tears or Saliva to Treat Eye or Mouth Diseases
Outlook
In this way, Yingli Wang and colleagues displayed a wearable plasmonic-electronic integrated sensor as a next-generation wearable device. When compared to existing wearable electrochemical sensors, this sensor showed broader target specificity and higher stability. The integrated device bridged the existing gap in personalized diagnosis and precision medicine to track important molecules inside the body in real time. The team proposed applications to monitor physiological cues and drug concentrations in a closed-loop feedback drug delivery system and expect the wearable sensor to inspire a range of multidisciplinary applications.