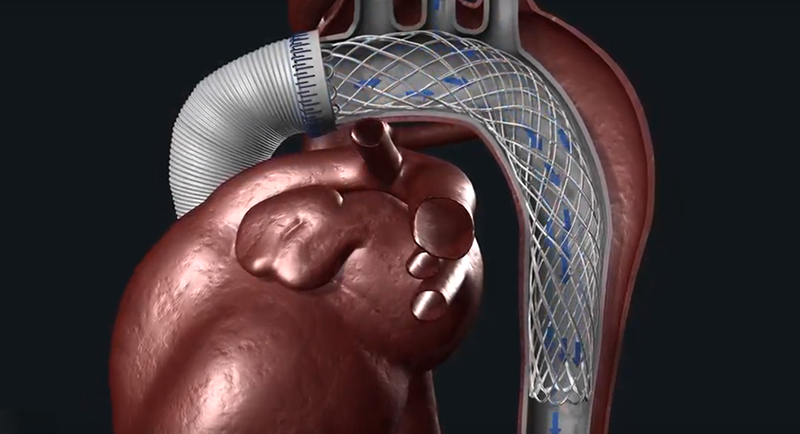
An acute type A aortic dissection is a life-threatening condition that requires significant surgical skills and a complicated decision-making process. Today’s surgical treatment, although lifesaving, does not effectively treat malperfusion and leaves the aorta untreated beyond the ascending segment, which can lead to negative remodeling, reoperations, and increased risk of mortality.
Related This “Smart Stent” Can Monitor Blood Flow and Detect Narrowing of Artery
Ascyrus Medical, a Boca Raton, FL-based medical device company, has now received CE mark approval for its Ascyrus Medical Dissection Stent (AMDS) for treatment of acute type A aortic dissections, according to a press release.
The AMDS is an arch remodeling hybrid graft designed as an adjunct to the current surgical reconstruction. The AMDS helps reduce the risk of complications and reoperations by treating malperfusion and stabilizing or reducing the size of the aorta, known as positive aortic remodeling.
The AMDS was given CE Mark approval after the success of DARTS I trial. DARTS, the largest prospectively controlled device trial for treatment of type A dissections, successfully demonstrated effective single- stage malperfusion treatment, positive remodeling, and improved survival vs. standard of care historical controls.
“By reducing the complications and reinterventions associated with acute aortic dissections, the AMDS significantly improves the care of patients far beyond what we have been able to accomplish prior to the AMDS therapy,” said Dr. Michael Moon, Cardiac Surgeon at the University of Alberta Edmonton and National Principal Investigator for DARTS.
Earlier this week, Ascyrus presented results from the DARTS I Trial at the 55th Annual STS meeting in San Diego. The results showed the AMDS effectively treated malperfusion in over 95% of cases. A unique characteristic in this cohort in addition to recovery of visceral vessels was healing of malperfusions involving cerebral vessels and reversal of dissection-induced paralysis.
Related Leviticus Cardio and Jarvik Heart Unveil Groundbreaking Wirelessly Powered Heart Pump
“We continue to be impressed with the clinical outcomes of the AMDS device and are thrilled with this milestone achievement of CE Mark approval. We look forward to partnering with hospitals and physicians throughout Europe to bring this lifesaving technology to more patients world-wide,” said Ali Shahriari, MD, Founder and CEO of Ascyrus Medical.