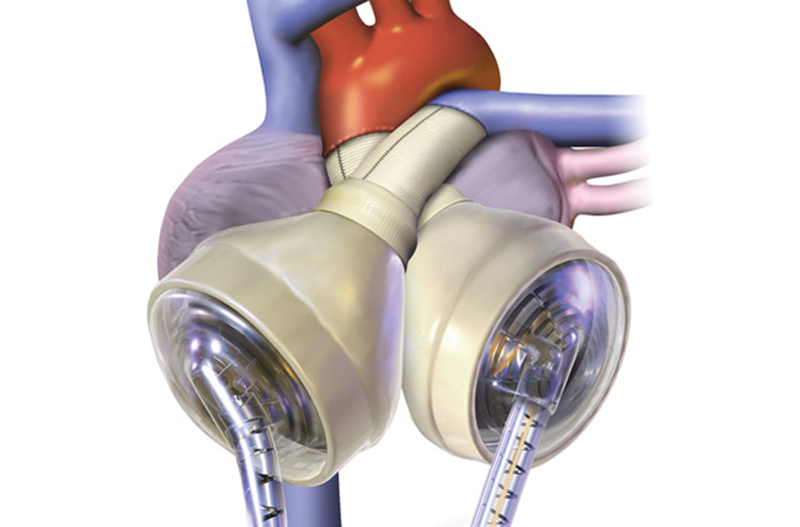
The SynCardia™ is the world’s only FDA-approved total artificial heart (TAH). This temporary heart is a pneumatically driven, pulsatile system capable of flows of more than 9 L/min.
If you or a loved one has end-stage biventricular heart failure, there’s hope. When a donor heart isn’t an available option, the SynCardia temporary Total Artificial Heart can provide the new heart you need without the wait. The SynCardia temporary Total Artificial Heart is a proven, life-saving treatment option for patients with end-stage biventricular failure.
The TAH is meant to be used as a bridge to transplantation (BTT) in patients at imminent risk of death from non-reversible bi-ventricular failure.
Just like a human heart, the SynCardia TAH is pulsatile and consists of two artificial ventricles made from biocompatible plastic, which prevents the TAH from being rejected by the body. And, the four valves pump blood throughout the body. The biocompatible plastic offers a high degree of fatigue resistance and strength for long-term durability.
The ventricles are connected together by Velcro, which allows the surgeon to position the ventricles in the chest based on the patient’s anatomy.
The Study
To further understand the efficiency of TAH, a study was conducted where the researchers:
- Used a closed loop platelet activity state assay to evaluate the degree of platelet reactivity induced by the TAH
- Modeled the motion of the TAH pulsatile mobile diaphragm
- Performed fluid-structure interactions and assessment of the flow behavior through inflow and outflow regions of the TAH fitted with modern bi-leaflet heart valves
Results
The researchers concluded that SynCardia TAH is a pneumatically driven pulsatile biventricular replacement device that has demonstrated clear life-saving efficacy in more than 1000 human implants to date.
Read more Japanese scientists Develop Solar-Powered Wearable Heart Monitor That Can Be Taped to Skin
The research team believes that with increasing adoption of this technology, it may be even further improved through thromboresistance optimization. Additionally, the device could be designed to be smaller in size and use mechanical valves. They said the device thrombogencity emulation (DTE) methodology is an effective means of rapidly extending and improving the TAH design to create smaller, durable, TAH systems with minimized overall thrombogenicity.
Developing a range of TAH devices will afford biventricular replacement therapy to a wide range of patients, for both short and long-term therapy.
About SynCardia Systems LLC
Headquartered in Tucson, Arizona, SynCardia was founded in 2001 by renowned cardiothoracic surgeon Jack G. Copeland, MD; biomedical engineer Richard G. Smith, MSEE, CCE; and interventional cardiologist Marvin J. Slepian, MD. Today, SynCardia is the sole manufacturer and provider of the world’s only commercially approved total artificial heart. In clinical use for more than 35 years, the SynCardia temporary Total Artificial Heart (TAH) is the most widely used and extensively studied total artificial heart in the world.