In recent years, wearable devices have become a part of the healthcare industry. According to Healthcare Information and Management Systems Society, Inc. (HIMSS), wearable technologies can be innovative solutions for healthcare problems, enable the continuous monitoring of human physical activities and behaviors, as well as physiological and biochemical parameters during daily life.
Read more The Role Technology is Playing to Stem the Tide of COVID-19
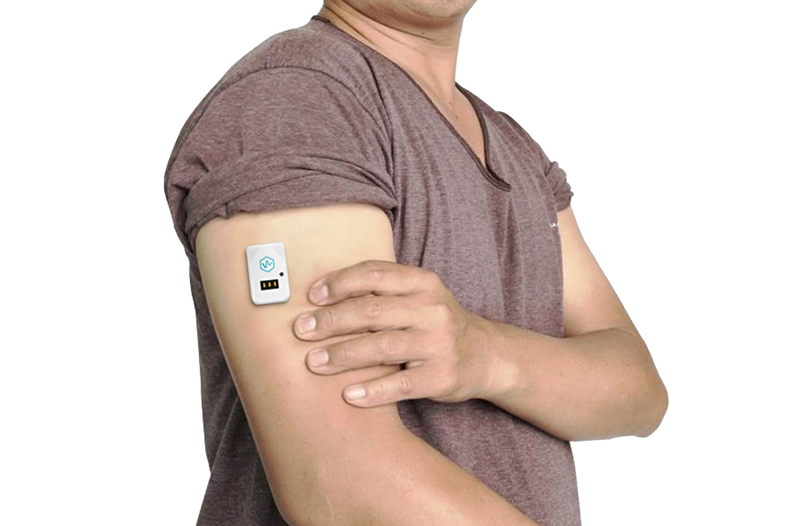
Wearable devices have started to play an important role in the healthcare industry even before the pandemic. Nowadays, during a severe viral pandemic, remote monitoring medical devices have become a crucial component of healthcare. In fact, earlier in March, the U.S. Food and Drug Administration (FDA) allowed certain FDA-cleared non-invasive, vital sign-measuring devices to expand their use so that health care providers can use them to monitor patients remotely, says a press release.
Nemaura Medical, announced breaking news last week that it has, “issued a presentation outlining how CGM is being used as an effective tool for the monitoring of disease progression in both quarantined and hospitalized COVID-19 patients. This includes improvement in glycemic control in persons with Type 2 diabetes, monitoring and managing hyperglycemia in patients with COVID-19, and remote monitoring of glucose levels in hospitalized COVID-19 patients leading to improved quality of care without compromising the safety of medical professionals. Both the CGM and CLM (continuous lactate monitoring) products are based on Nemaura’s BEAT™ platform, which is designed to non-invasively extract a number of analytes through the skin.
Inovio Pharmaceuticals announced back in May the publication of the preclinical study data for IN0-4800, its COVID-19 DNA vaccine, demonstrating robust neutralizing antibody and T cell immune responses against coronavirus SARS-CoV-2. The study was published in the peer-reviewed journal Nature Communications.

BioNTech announced this month that the first 72 participants have already been dosed with BNT162b1 following IND approval by the Chinese regulatory authority, National Medical Products Administration (NMPA). BioNTech and Fosun Pharma are jointly developing the COVID-19 vaccine candidate in China. The trial is part of BioNTech’s global development program aimed at supporting a global supply upon regulatory approval.
GlaxoSmithKline plc and Vir Biotechnology announced back in April that they have signed a binding agreement to enter into a collaboration to research and develop solutions for coronaviruses, including SARS-CoV-2, the virus that causes COVID-19. The collaboration will use Vir’s proprietary monoclonal antibody platform technology to accelerate existing and identify new anti-viral antibodies that could be used as therapeutic or preventative options to help address the current COVID-19 pandemic and future outbreaks. The companies will leverage GSK’s expertise in functional genomics and combine their capabilities in CRISPR screening and artificial intelligence to identify anti-coronavirus compounds that target cellular host genes. They will also apply their combined expertise to research SARS-CoV-2 and other coronavirus vaccines.
Eli Lilly and Company announced earlier this month the initiation of BLAZE-2, a Phase 3 trial studying LY-CoV555 for the prevention of SARS-CoV-2 infection and COVID-19 in residents and staff at long-term care facilities in the U.S. (skilled nursing facilities, commonly referred to as nursing homes, and assisted living facilities). LY-CoV555, the lead antibody from Lilly’s collaboration with AbCellera, is a neutralizing antibody against SARS-CoV-2, the virus that causes COVID-19.