Robocath’s Robotic-Assisted Solution R-One Gets CE Mark for Treating Coronary Diseases
Robocath, a Rouen, France-based company that develops robotic systems for treating cardiovascular diseases, won CE certification for its R-One™ robotic device. The CE approval...
Marvell, Samsung Extend Long-Term Partnership for 5G Wireless Networks
Marvell, a leading infrastructure semiconductor solutions provider, expanded it’s a long-term partnership with Samsung for enabling leading wireless infrastructure networks. The two companies are...
STATS’ AI-Powered Technology AutoSTATS Allows Capturing of Sports Tracking Data via Broadcast Video
STATS, a Chicago-based sports data, technology, statistics, and content company, officially launched AutoSTATS, the first artificial intelligence (AI) and computer vision technology to deliver...
Verisense™ Wearable Sensor Platform for Clinical Trials – Interview with Geoff Gill, president of...
Shimmer, a global leader in wearable technology for research applications, has just launched a next-generation wearable sensor platform. We interviewed Geoff Gill, the president...
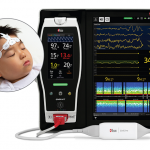
Masimo Receives CE Marking for Next Gen SedLine Brain Function Monitoring for Pediatric Patients
Masimo, an Irvine, California-based manufacturer of noninvasive patient monitoring technologies, received CE marking for its next generation SedLine® brain function monitoring for pediatric patients....
Navitian, iVascular´s New Coronary Microcatheter Receives CE Mark Approval
iVascular, a Barcelona, Spain-based catheter maker, won CE Mark for its new microcatheter called Navitian. The microcatheter can be used to facilitate, guide and...