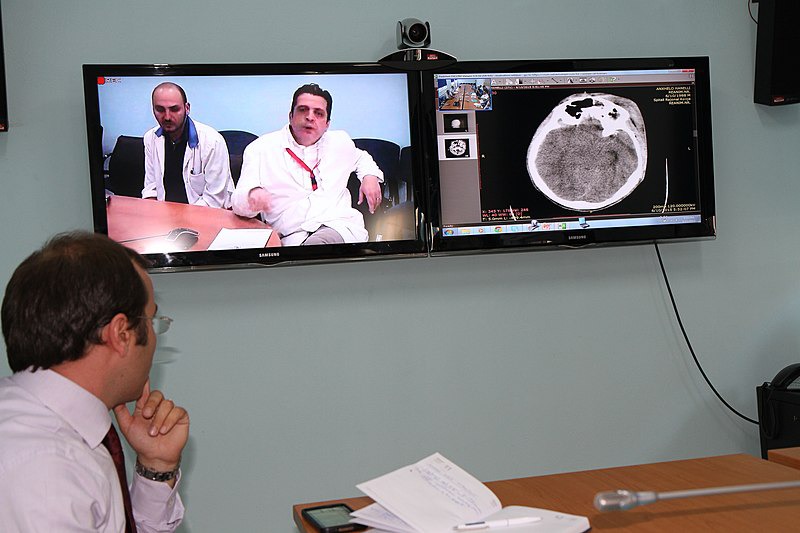
Telemedicine, also known as e-medicine, is the remote delivery of healthcare services, including tests and consultations, over the telecommunications infrastructure. Telemedicine allows health care professionals to evaluate, diagnose and treat patients at a distance using telecommunications technology.
For telemedicine to be truly useful, the patient must be able to collect and transmit a variety of data the healthcare professional needs in order to assess the patient’s health, writes Bradley Merrill Thompson in MobileHealthNews.
Artificial intelligence (AI) can help with data collection by instructing patients how to use medical technology that collects data. AI can help physicians sort through and analyze the data they receive, and it can even help deliver therapy.
Read more COVID-19 Pandemic Will Propel US Telehealth Market To Grow At A CAGR of Over 29% During 2019-25
In April 2019, the FDA published a request for comments on new proposals for the regulation of medical devices that employ artificial intelligence (AI) and machine learning (ML) components.
“Artificial intelligence and machine learning technologies have the potential to transform health care by deriving new and important insights from the vast amount of data generated during the delivery of health care every day. Medical device manufacturers are using these technologies to innovate their products to better assist health care providers and improve patient care. The FDA is considering a total product lifecycle-based regulatory framework for these technologies that would allow for modifications to be made from real-world learning and adaptation, while still ensuring that the safety and effectiveness of the software as a medical device is maintained,” The FDA said.
In the face of COVID-19 with its attendant greater need for medical services, while achieving social distancing, it seems that FDA is now ready to support the use of AI in telemedicine.

In February of this year, FDA authorized the marketing of AI that would guide cardiac ultrasound use. In its first FDA authorization, this guidance system is limited to use by healthcare professionals, but fundamentally the technology now exists to use AI to guide the use of historically complex diagnostic tools.
FDA seems to be doing what it can to encourage the development and deployment of these devices on the patient side to make telemedicine more powerful.
In its Policy for Imaging Systems Used During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency, FDA notes that “Imaging devices can help visualize pulmonary abnormalities and are used routinely to diagnose and evaluate the causes of reduced lung function. Accordingly, there is an increased demand for imaging devices that may assist in the diagnosis and treatment monitoring of lung disease.” FDA further explains “Increasing the availability of mobile and portable systems may increase options to image patients inside and outside of healthcare facilities, which could help to reduce the spread of COVID-19. Additionally, modified use of ultrasound imaging systems may expand the number of healthcare practitioners capable of performing this imaging technique.”
Read more Theranica Partners With Cove to Expand Availability of Nerivio on Cove’s Telemedicine Platform
Apart from image analysis, FDA has come out with a new redraft of its Clinical Decision Support Software Guidance last September that is much more flexible in its treatment of AI.
“FDA has provided a road map for guidance-system technologies in ways that they can be used to facilitate telemedicine. Unfortunately this roadmap can’t be found in any guidance, and no doubt the rules will continue to evolve. But it appears that FDA is willing to be creative and flexible. And, nearly all of this was even before COVID-19 created an urgency around the use of telemedicine. The future is looking pretty bright for these technologies,” Thompson wrote in his report.